Going Grassroots: Dr. Unger on the CDC's Chronic Fatigue Syndrome Multisite Studies
The CDC's Chronic Fatigue Syndrome Multisite Studies
The CDC multisite studies will hopefully change the way physicians think about Chronic Fatigue Syndrome, identify subsets, open up treatment options, provide outcome measures for clinical trials, validate the significance of post-exertional malaise and basically help legitimize ME/CFS. That's a lot to ask, but then again these are large studies, the likes of which we've never seen before. The stakes are pretty high for Dr. Unger's CFS team. They haven't exactly been lighting up the research world with their ME/CFS publications. The last one - on a patient registry - was published over a year ago and publications were pretty spotty before that. The last really interesting CDC study was a gynecologic study published in 2011. One ME/CFS expert bluntly told me "Don't expect breakthroughs from the CDC - that's not what they do".Dr. Unger has stated the CDC's CFS team is devoting most of their energy to the multicenter studies. If major insights don't come out of them quite a few people are going to be surprised and disappointed. They should start appearing in the not too distant future.To get ready for that, first-we check out an ME/CFS Alert interview with someone who was smack dab in the middle of all the moving pieces - former Simmaron research assistant Gunnar Gottschalk - and then present an interview with Dr. Unger of the CDC about the studies and other matters.
The stakes are pretty high for Dr. Unger's CFS team. They haven't exactly been lighting up the research world with their ME/CFS publications. The last one - on a patient registry - was published over a year ago and publications were pretty spotty before that. The last really interesting CDC study was a gynecologic study published in 2011. One ME/CFS expert bluntly told me "Don't expect breakthroughs from the CDC - that's not what they do".Dr. Unger has stated the CDC's CFS team is devoting most of their energy to the multicenter studies. If major insights don't come out of them quite a few people are going to be surprised and disappointed. They should start appearing in the not too distant future.To get ready for that, first-we check out an ME/CFS Alert interview with someone who was smack dab in the middle of all the moving pieces - former Simmaron research assistant Gunnar Gottschalk - and then present an interview with Dr. Unger of the CDC about the studies and other matters.
Gunnar Gottschalk on on the Simmaron Research Foundation and the CDC's Multisite ME/CFS Study
Background - The Open Medicine Institute Opens the Door
Dr. Kogelnik with his double PhD in medicine and computer science is doing his best to usher the ME/CFS field into a new age. Kogelnik believes lots of data is going to be needed to understand Chronic Fatigue Syndrome. In order to get it he struck a deal with a network of top ME/CFS physicians. He would give them electronic data bases and show them how to use them and they would provide him with numerically identified data (no names) he could use to assist his research. Everybody won. With their almost 40,000 charts the Simmaron Research Foundation and Sierra Internal Medicine (Dr. Peterson’s practice) had been almost drowning in immune data. It took hundreds of hours to get the data processed, but whereas accessing that data before required a chart by chart review now it simply requires telling the computer to search for, say, NK cell functioning test results. The updated approach laid the foundation for Dr. Peterson and the Simmaron Research Foundation to participate in research studies with top researchers. It also opened the door for the multisite studies.When some money got freed up at the end of the year Dr. Unger asked for proposals. Kogelnik jumped at the chance and submitted a grant for a multisite study examining patients at ME/CFS expert’s practices across the country. Unger snatched up Kogelnik’s proposal and added more sites and the multisite study, now the main focus of the CDC’s ME/CFS research group, was born.
Everybody won. With their almost 40,000 charts the Simmaron Research Foundation and Sierra Internal Medicine (Dr. Peterson’s practice) had been almost drowning in immune data. It took hundreds of hours to get the data processed, but whereas accessing that data before required a chart by chart review now it simply requires telling the computer to search for, say, NK cell functioning test results. The updated approach laid the foundation for Dr. Peterson and the Simmaron Research Foundation to participate in research studies with top researchers. It also opened the door for the multisite studies.When some money got freed up at the end of the year Dr. Unger asked for proposals. Kogelnik jumped at the chance and submitted a grant for a multisite study examining patients at ME/CFS expert’s practices across the country. Unger snatched up Kogelnik’s proposal and added more sites and the multisite study, now the main focus of the CDC’s ME/CFS research group, was born.
The Sites
- Dr Lucinda Bateman (Fatigue Consultation Clinic, UT)
- Dr. Nancy Klimas (Center for Neuro Immune Medicine, FL)
- Dr Andreas Kogelnik (Open Medicine Clinic, CA)
- Dr Charles Lapp (Hunter-Hopkins Center, NC)
- Dr. Benjamin Natelson (Pain and Fatigue Study Center, NY)
- Dr Daniel Peterson (Sierra Internal Medicine Associates, NV)
- Dr Richard Podell (Richard Podell Clinic, NJ)
 Gunnar stated the motto behind the study was getting the CDC on the ground grass-roots style and take close look at ME/CFS patients. The multisite study is going to give the CDC, he said, a sense of what this illness really is. What are the primary symptoms? Do you have a history of viral infection or autoimmune disorder? What illnesses run in the family? How sick are these patients? How long have they been sick? What do they test positive for? (What do they test negative for?)For the first time the medical world and the CDC is going to view Chronic Fatigue Syndrome patients, not through the filter of the CDC’s Toolkit or the dumbed down sites on the Mayo Clinic or WebMD, but through the eyes of expert practitioners trying to help them.
Gunnar stated the motto behind the study was getting the CDC on the ground grass-roots style and take close look at ME/CFS patients. The multisite study is going to give the CDC, he said, a sense of what this illness really is. What are the primary symptoms? Do you have a history of viral infection or autoimmune disorder? What illnesses run in the family? How sick are these patients? How long have they been sick? What do they test positive for? (What do they test negative for?)For the first time the medical world and the CDC is going to view Chronic Fatigue Syndrome patients, not through the filter of the CDC’s Toolkit or the dumbed down sites on the Mayo Clinic or WebMD, but through the eyes of expert practitioners trying to help them.
Standardized Approach Aids Subset Search
The doctors came together with the CDC to produce a standardized intake form which included newer, simpler ways of measuring fatigue and other symptoms.(The group clearly was not satisfied with the current symptom assessment tools used in ME/CFS. The AHRQ panel was not particularly satisfied with them either and reported that no validated tools exist for ME/CFS. That’s a big problem. Somehow you have to find a way to measure fatigue, even if it’s only to determine that a biomarker is actually a biomarker.) They also agreed to a standardized testing protocol. Before the study these practitioners used different tests to assess the health of their patients. They still do their own tests, but now all patients in the study get a core set of tests. Dr. Peterson’s patients,for instance, will get a comprehensive immune workup that includes everything from NK cell numbers and function and IgG subclasses to clonal T-cell rearrangements, but they'll also get a standard set of tests.This group, then, did two things right off the bat the AHRQ panel was calling for; they developed (hopefully) better symptom assessment tests and agreed to a standardized testing protocol.If subsets don’t pop out after all this everyone is going to be surprised. Besides characterizing the patients in every way possible (symptoms, disease history, medication use, treatment outcomes, test results) the study may help develop outcome measures for clinical trials. Developing “outcome measures” – standardized tests drug companies could rely on to determine if their products were making a difference in ME/CFS – would be a big deal.The CDC will also continue to follow up the patients over time to determine how the disease proceeds.
They also agreed to a standardized testing protocol. Before the study these practitioners used different tests to assess the health of their patients. They still do their own tests, but now all patients in the study get a core set of tests. Dr. Peterson’s patients,for instance, will get a comprehensive immune workup that includes everything from NK cell numbers and function and IgG subclasses to clonal T-cell rearrangements, but they'll also get a standard set of tests.This group, then, did two things right off the bat the AHRQ panel was calling for; they developed (hopefully) better symptom assessment tests and agreed to a standardized testing protocol.If subsets don’t pop out after all this everyone is going to be surprised. Besides characterizing the patients in every way possible (symptoms, disease history, medication use, treatment outcomes, test results) the study may help develop outcome measures for clinical trials. Developing “outcome measures” – standardized tests drug companies could rely on to determine if their products were making a difference in ME/CFS – would be a big deal.The CDC will also continue to follow up the patients over time to determine how the disease proceeds.
Expansion - the Second Phase of the Multisite Study
The first phase of the multisite study has been finished and we’re awaiting publication of the results. The CDC study was already very large – 75 patients at each of the 8 sites – and in the second phase, if I have it right, it gets much larger (150 persons at each site.) If those numbers are correct this is easily the biggest ME/CFS study ever undertaken.Pediatric patients, healthy controls and people with similar illnesses are being added to the second phase of the study and biological data will be collected specifically for the study for the first time.That biological data includes salivary cortisol awakening levels, gene expression and lactate levels before and after a 1-day exercise test and ‘exercise capacity’. The blood will be banked to aid future research efforts.
Interlude: The Simmaron Research Foundation’s BioBank Multiple Sclerosis Study
Gunnar spoke about the Simmaron Research Foundation’s BioBank. The BioBank doesn’t get mentioned a lot, but with 6,000 specimens it’s one of the largest in existence and with samples dating back decades it’s certainly the oldest. The jewel in its crown are its unusually large number of the spinal fluid samples – something Dr. Mady Hornig has called ‘a treasure’.The Simmaron Research Foundation contracted with the Chronic Fatigue Initiative to analyze 60 of those samples and compare them to people with multiple sclerosis and healthy controls. That study got more interesting when a recent study found very low levels of a neuronal repair agent called BDNF in both multiple sclerosis and ME/CFS patients.
A Talk with Dr. Unger
"Publishing this information is a priority"
 You’ve done a lot of outreach including visiting the clinics involved in the multi-site study. Can you say one thing that surprised you or stuck in your mind from your visits to the different clinics?It was really a pleasure visiting the clinics and meeting those who care for CFS patients and work so hard on making sure the study is a success. While the style of each clinic varied, I was impressed by the care taken to make the waiting and examination rooms accommodate the special needs of CFS patients. In each clinic, the staff provided positive energy and empathy for their patients and it was clear how important these intangible items are to the patients. The experience emphasizes the need for us to work towards the time when all CFS patients will have access to the level of care provided in these clinics.Preliminary information on the CDC multi-site studies for CFS was provided at an FDA meeting a year and a half ago, but no studies have been published and no presentations on the studies were given at the IACFS/ME conference. Can you tell us roughly when the studies will start to be published. What subjects will the published studies be on? At the time when abstracts for IACFS/ME were due, the interim analysis presented at the FDA meeting was publicly available and therefore not appropriate to submit as new information. We were in the process of finalizing and verifying (sometimes called “cleaning”) the final dataset for the baseline year of enrollment into the multi-site study.We are now conducting analyses on these data and will write the papers and submit them to peer reviewed journals as soon as we can. The first paper will describe the characteristics of patients and their illness as measured by the questionnaires they completed. All patients enrolled in the baseline study will be included.Subsequent papers will describe additional features, such as medication use and medical history. The total time to write, clear, submit and have the articles peer reviewed is difficult to estimate. Publishing this information is a priority, and we will move this process along as quickly as possible.
You’ve done a lot of outreach including visiting the clinics involved in the multi-site study. Can you say one thing that surprised you or stuck in your mind from your visits to the different clinics?It was really a pleasure visiting the clinics and meeting those who care for CFS patients and work so hard on making sure the study is a success. While the style of each clinic varied, I was impressed by the care taken to make the waiting and examination rooms accommodate the special needs of CFS patients. In each clinic, the staff provided positive energy and empathy for their patients and it was clear how important these intangible items are to the patients. The experience emphasizes the need for us to work towards the time when all CFS patients will have access to the level of care provided in these clinics.Preliminary information on the CDC multi-site studies for CFS was provided at an FDA meeting a year and a half ago, but no studies have been published and no presentations on the studies were given at the IACFS/ME conference. Can you tell us roughly when the studies will start to be published. What subjects will the published studies be on? At the time when abstracts for IACFS/ME were due, the interim analysis presented at the FDA meeting was publicly available and therefore not appropriate to submit as new information. We were in the process of finalizing and verifying (sometimes called “cleaning”) the final dataset for the baseline year of enrollment into the multi-site study.We are now conducting analyses on these data and will write the papers and submit them to peer reviewed journals as soon as we can. The first paper will describe the characteristics of patients and their illness as measured by the questionnaires they completed. All patients enrolled in the baseline study will be included.Subsequent papers will describe additional features, such as medication use and medical history. The total time to write, clear, submit and have the articles peer reviewed is difficult to estimate. Publishing this information is a priority, and we will move this process along as quickly as possible.
Treatment and Testing
"The long-term goal is to identify biologically meaningful subgroups so that the design of treatment trials could be refined, both in terms of those enrolled and outcomes measured"
Are you examining treatment effects in the second part of the study? As patients return to the clinics, we are collecting follow-up information about the characteristics of their illness. We plan on doing this over several years to get an idea of whether/how their illness changes with time. This information, often referred to as “disease course,” will reflect both treatment effects and the natural history of the illness. We are collecting information on medications and therapy, so a correlation between disease course and treatment could be made.However, information from this observational study (i.e., a study that collects data without introducing a specific intervention) will provide only indirect evidence of treatment effects.[This excellent news indicates that the second part of the study will, for the first time ever, report not just on the wide variety of treatments used by ME/CFS experts, but on their efficacy. The report will lack the weight of an interventional study but documenting the efficacy of the wide range of treatment approaches – from antivirals to beta blockers to blood volume enhancers – should open eyes in the medical community.]Could this part of the study be used to broaden physician understanding of the appropriate diagnostic tests for Chronic Fatigue Syndrome? Do you expect that the CDC might, based on the data collected, update their recommendations for diagnostic testing in the Toolkit? Summarizing the tests used by our clinicians is only one step in understanding the best diagnostic practices for CFS. We hope that this will form the basis for dialogue between clinicians about the rationale for each test and how they use the test results to improve treatment. This study information, along with other evidence-based research, may enable updates to future CFS educational materials.
As patients return to the clinics, we are collecting follow-up information about the characteristics of their illness. We plan on doing this over several years to get an idea of whether/how their illness changes with time. This information, often referred to as “disease course,” will reflect both treatment effects and the natural history of the illness. We are collecting information on medications and therapy, so a correlation between disease course and treatment could be made.However, information from this observational study (i.e., a study that collects data without introducing a specific intervention) will provide only indirect evidence of treatment effects.[This excellent news indicates that the second part of the study will, for the first time ever, report not just on the wide variety of treatments used by ME/CFS experts, but on their efficacy. The report will lack the weight of an interventional study but documenting the efficacy of the wide range of treatment approaches – from antivirals to beta blockers to blood volume enhancers – should open eyes in the medical community.]Could this part of the study be used to broaden physician understanding of the appropriate diagnostic tests for Chronic Fatigue Syndrome? Do you expect that the CDC might, based on the data collected, update their recommendations for diagnostic testing in the Toolkit? Summarizing the tests used by our clinicians is only one step in understanding the best diagnostic practices for CFS. We hope that this will form the basis for dialogue between clinicians about the rationale for each test and how they use the test results to improve treatment. This study information, along with other evidence-based research, may enable updates to future CFS educational materials.
"One of our goals is to use data from this multi-site study to help refine sub-groups of CFS"
I see that lab test data is being gathered for the multi-site study. Does that mean we can expect a study at some point indicating what percentage of people with Chronic Fatigue Syndrome at these clinics test positive for, say, IgG subclass deficiencies or orthostatic intolerance or even reduced aerobic capacity (understanding that not all practitioners test for them)? We have included a record review of testing in order to provide information on the different kinds of tests that CFS expert clinicians find helpful in managing CFS patients. Because testing is not uniform (not all clinicians order the same tests for their patients) and not random (testing is guided by the individual patient’s symptoms), test results from record review will not be a good estimate of the percentage of CFS patients with test abnormalities. Tests that are being done as part of the study, such as a measure of orthostatic intolerance that is part of the physical examination or wakening cortisol response, should be available for most patients and will be reported.In an earlier interview you stated that "The study is expected to provide data to support new initiatives throughout the CFS research community." What kind of new initiatives do you expect the study might support?One of our goals is to use data from this multi-site study to help refine sub-groups of CFS and provide information on reliability of measures of illness. Findings from this study will require validation by others. Similarly, the validity of patient subgroups suggested by other studies could be tested in our data.The long-term goal is to identify biologically meaningful subgroups so that the design of treatment trials could be refined, both in terms of those enrolled and outcomes measured. In addition, the biorepository we are building will be linked to carefully curated patient data. This could be used by researchers to refine or validate promising biomarkers or disease hypotheses.
We have included a record review of testing in order to provide information on the different kinds of tests that CFS expert clinicians find helpful in managing CFS patients. Because testing is not uniform (not all clinicians order the same tests for their patients) and not random (testing is guided by the individual patient’s symptoms), test results from record review will not be a good estimate of the percentage of CFS patients with test abnormalities. Tests that are being done as part of the study, such as a measure of orthostatic intolerance that is part of the physical examination or wakening cortisol response, should be available for most patients and will be reported.In an earlier interview you stated that "The study is expected to provide data to support new initiatives throughout the CFS research community." What kind of new initiatives do you expect the study might support?One of our goals is to use data from this multi-site study to help refine sub-groups of CFS and provide information on reliability of measures of illness. Findings from this study will require validation by others. Similarly, the validity of patient subgroups suggested by other studies could be tested in our data.The long-term goal is to identify biologically meaningful subgroups so that the design of treatment trials could be refined, both in terms of those enrolled and outcomes measured. In addition, the biorepository we are building will be linked to carefully curated patient data. This could be used by researchers to refine or validate promising biomarkers or disease hypotheses.
The Exercise Study
"PEM is just one of the factors that could be used to stratify patients"
There is fatigue and there is post-exertional malaise. For myself the inability to do even mild exercise without experiencing significant symptoms is the outstanding aspect of ME/CFS. Everything else is kind of nebulous; the fatigue, the body pains, the cognitive problems - but ask me to exercise and everything gets much worse very quickly. It's hard for me and others to imagine, therefore, that PEM is not the most biologically significant symptom in this illness, but some people who meet the CFS criteria are significantly fatigued but don't appear to experience PEM. I have two questions(1) Don't we run the risk that we are mixing apples and oranges - and thus doing injustice to both - by not separating these two groups in research studies? (2) Will the multi-site study help us understand these two groups better? Patient heterogeneity is a problem for research studies. PEM is just one of the factors that could be used to stratify patients. Type of onset (acute versus gradual), severity of individual symptoms, and cognitive measures are some other examples. Information from the multi-site study will hopefully start the process of identifying biologically meaningful subgroups. When we have good ways to look at all the components of the illness, studies can then be designed to look at subgroups. [Commentary: Dr. Unger has not been convinced in the past that post-exertional malaise is a defining characteristic of ME/CFS or “ME” and she clearly is not now. The idea that PEM is one factor among many has been the CDC’s stance for some time.The fact that Dr. Unger is using an exercise test of all things to reveal the biological breakdowns present in this disorder indicates that she recognizes how important a role exertion plays in this illness, but she believes that stratifying patients according to disease onset, cognition or symptoms severity might produce as biologically meaningful subsets.That's questionable. Because many different processes undoubtedly contribute to symptom severity in ME/CFS, focusing on that seems unlikely point a finger at a specific disease process. Nor have differences in cognition (working memory problems vs say information processing speed) ever been suggested as being possible differentiating factors in ME/CFS. Disease onset is a more likely candidate but most studies that have examined disease onset have not found it to be a significant factor.The Light ME/CFS multiple sclerosis study suggested that the PEM induced fatigue and pain is different physiologically from the "lassitude" type of fatigue in MS. If PEM is a more or less unique contributor to fatigue in ME/CFS it would provide an important tool to differentiate ME/CFS from other disorders. It's clear that "fatigue" is too crude of a concept to denote what's happening in ME/CFS.Dr. Unger ‘s assertion that when we have ‘good ways to look at all components’ of ME/CFS then we should look for subgroups was echoed, to some extent, by the AHRQ in their report to the P2P panel. That we still, after thirty years, don’t have the tools to properly assess the symptom presentation in ME/CFS is nauseating, but simply reflects the lack of federal support for this field. The NIH’s willingness to point out the flaws in ME/CFS research has never, unfortunately, coincided with a willingness to spend any money to fix them.]One report suggested the CDC was gathering data on lactic acid accumulation during exercise. Is this so? We are measuring lactate during the exercise test that is being conducted at several of the clinical sites as part of the combined cognition-exercise protocol. Lactate is measured at the start of exercise and periodically throughout the test (including peak exercise intensity) and during exercise recovery.This information will be used along with other measures taken during the test (gas exchange data) to assist with determination of the exercise capacity of participants.
[Commentary: Dr. Unger has not been convinced in the past that post-exertional malaise is a defining characteristic of ME/CFS or “ME” and she clearly is not now. The idea that PEM is one factor among many has been the CDC’s stance for some time.The fact that Dr. Unger is using an exercise test of all things to reveal the biological breakdowns present in this disorder indicates that she recognizes how important a role exertion plays in this illness, but she believes that stratifying patients according to disease onset, cognition or symptoms severity might produce as biologically meaningful subsets.That's questionable. Because many different processes undoubtedly contribute to symptom severity in ME/CFS, focusing on that seems unlikely point a finger at a specific disease process. Nor have differences in cognition (working memory problems vs say information processing speed) ever been suggested as being possible differentiating factors in ME/CFS. Disease onset is a more likely candidate but most studies that have examined disease onset have not found it to be a significant factor.The Light ME/CFS multiple sclerosis study suggested that the PEM induced fatigue and pain is different physiologically from the "lassitude" type of fatigue in MS. If PEM is a more or less unique contributor to fatigue in ME/CFS it would provide an important tool to differentiate ME/CFS from other disorders. It's clear that "fatigue" is too crude of a concept to denote what's happening in ME/CFS.Dr. Unger ‘s assertion that when we have ‘good ways to look at all components’ of ME/CFS then we should look for subgroups was echoed, to some extent, by the AHRQ in their report to the P2P panel. That we still, after thirty years, don’t have the tools to properly assess the symptom presentation in ME/CFS is nauseating, but simply reflects the lack of federal support for this field. The NIH’s willingness to point out the flaws in ME/CFS research has never, unfortunately, coincided with a willingness to spend any money to fix them.]One report suggested the CDC was gathering data on lactic acid accumulation during exercise. Is this so? We are measuring lactate during the exercise test that is being conducted at several of the clinical sites as part of the combined cognition-exercise protocol. Lactate is measured at the start of exercise and periodically throughout the test (including peak exercise intensity) and during exercise recovery.This information will be used along with other measures taken during the test (gas exchange data) to assist with determination of the exercise capacity of participants. [One of concerns regarding using the one-day aerobic exercise test to measure ‘exercise capacity’ is that studies indicate that a two-day exercise test in ME/CFS is required to pick significant decrements in aerobic exercise capacity. The one-day test then, is a poor and misleading measure of exercise capacity in ME/CFS. The CDC's use of the one-day test has lead to fears that the negative results will overshadow the two-day exercise studies that indicate ‘exercise capacity’ is, indeed blunted in ME/CFS.It’s not clear what Dr. Unger meant when she said the 1-day test will assist with determining the ‘exercise capacity’ (probably VO2 max, etc. and lactate) of ME/CFS patients. The lactate measurements taken during the test may demonstrate decrements in exercise capacity but past studies suggest aerobic capacity – the ability to generate energy - will largely appear normal in her group.If the study results mirror what Dane Cook in his Solve ME/CFS study are finding, however, the one-day test will reveal significant drops in cognitive functioning after exercise.]In that interview you also stated "We believe that the data collected in our study will help identify the best options for either measuring post-exertional malaise or for identifying measures that correlate with this characteristic." Could you expand on that a bit? What data might come out of the study that could help measure the extent of PEM present?We are asking participants in the exercise and cognition study to complete simple self-reported scales of physical fatigue, mental fatigue (or mental fog), muscle aches, joint aches, headache, muscle weakness and light headedness. In addition, we are asking participants to complete online cognitive tests after they return home. We hope that changes in these measures across multiple time points before, during, and after testing will provide insights to the extent of PEM.[It’s a bit mystifying that Dr. Unger did not include lactate or gene expression in her answer since she is measuring them in response to and after exercise as well, but perhaps the question was not phrased well. Differing gene expression results or lactate levels in ME/CFS patients and controls could surely help measure the extent of PEM present in ME/CFS. ]Do you see any possibility that we might identify some diagnostic factors that could predict the presence of PEM without having ME/CFS patients get on a bicycle? It is not clear whether there are diagnostic factors that correlate with PEM, but we will be evaluating all measures collected as part of the study. Ideally, we would like to have an alternative to formal exercise testing.Will the gene expression part of the study target the same genes that the Lights identified during their exercise studies? Will you be banking the blood collected during and after the exercise test so that it can be assessed again when future research suggests it might be appropriate to do that?We will have one blood sample collected before and one after exercise testing. RNA from whole blood can be tested for multiple genes including those identified in the Lights’ studies. The samples will be available to test different hypotheses.)
[One of concerns regarding using the one-day aerobic exercise test to measure ‘exercise capacity’ is that studies indicate that a two-day exercise test in ME/CFS is required to pick significant decrements in aerobic exercise capacity. The one-day test then, is a poor and misleading measure of exercise capacity in ME/CFS. The CDC's use of the one-day test has lead to fears that the negative results will overshadow the two-day exercise studies that indicate ‘exercise capacity’ is, indeed blunted in ME/CFS.It’s not clear what Dr. Unger meant when she said the 1-day test will assist with determining the ‘exercise capacity’ (probably VO2 max, etc. and lactate) of ME/CFS patients. The lactate measurements taken during the test may demonstrate decrements in exercise capacity but past studies suggest aerobic capacity – the ability to generate energy - will largely appear normal in her group.If the study results mirror what Dane Cook in his Solve ME/CFS study are finding, however, the one-day test will reveal significant drops in cognitive functioning after exercise.]In that interview you also stated "We believe that the data collected in our study will help identify the best options for either measuring post-exertional malaise or for identifying measures that correlate with this characteristic." Could you expand on that a bit? What data might come out of the study that could help measure the extent of PEM present?We are asking participants in the exercise and cognition study to complete simple self-reported scales of physical fatigue, mental fatigue (or mental fog), muscle aches, joint aches, headache, muscle weakness and light headedness. In addition, we are asking participants to complete online cognitive tests after they return home. We hope that changes in these measures across multiple time points before, during, and after testing will provide insights to the extent of PEM.[It’s a bit mystifying that Dr. Unger did not include lactate or gene expression in her answer since she is measuring them in response to and after exercise as well, but perhaps the question was not phrased well. Differing gene expression results or lactate levels in ME/CFS patients and controls could surely help measure the extent of PEM present in ME/CFS. ]Do you see any possibility that we might identify some diagnostic factors that could predict the presence of PEM without having ME/CFS patients get on a bicycle? It is not clear whether there are diagnostic factors that correlate with PEM, but we will be evaluating all measures collected as part of the study. Ideally, we would like to have an alternative to formal exercise testing.Will the gene expression part of the study target the same genes that the Lights identified during their exercise studies? Will you be banking the blood collected during and after the exercise test so that it can be assessed again when future research suggests it might be appropriate to do that?We will have one blood sample collected before and one after exercise testing. RNA from whole blood can be tested for multiple genes including those identified in the Lights’ studies. The samples will be available to test different hypotheses.)
Other CDC ME/CFS Research
"A review of the literature on CFS suggests that CFS shares some features with accelerated aging ..."
What was the rationale for the telomere length study? How does that fit in with past/future CDC research? 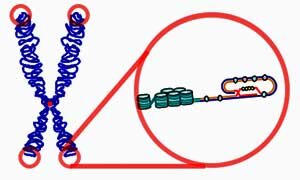 Your question about telomere length probably refers to information that Dr. Rajeevan presented at the 2014 IACFS/ME meeting. We conducted an analysis of telomere in archived samples from our population-based study of CFS. This was prompted because of the association of telomere length with aging, cancer, heart disease, infection and stress. A review of the literature on CFS suggests that CFS shares some features with accelerated aging and post-infective fatigue.Around the time that the laboratory had finished testing telomere length, we also completed the testing to identify genes that had different methylation patterns in CFS cases compared to non-fatigued controls. Samples in the methylation analysis were also part of the population study. The exploratory methylation testing identified several genes that differed in methylation, but only one gene that also showed a difference in expression. That gene was TERT (telomerase reverse transcriptase), an enzyme that is involved in maintaining telomeres. These observations need to be confirmed in other populations.Many factors that occur with CFS could contribute to both methylation and telomere length, so the findings are not clear. They do suggest that some of the same biologic factors that are seen in a variety of chronic conditions are also seen in CFS.
Your question about telomere length probably refers to information that Dr. Rajeevan presented at the 2014 IACFS/ME meeting. We conducted an analysis of telomere in archived samples from our population-based study of CFS. This was prompted because of the association of telomere length with aging, cancer, heart disease, infection and stress. A review of the literature on CFS suggests that CFS shares some features with accelerated aging and post-infective fatigue.Around the time that the laboratory had finished testing telomere length, we also completed the testing to identify genes that had different methylation patterns in CFS cases compared to non-fatigued controls. Samples in the methylation analysis were also part of the population study. The exploratory methylation testing identified several genes that differed in methylation, but only one gene that also showed a difference in expression. That gene was TERT (telomerase reverse transcriptase), an enzyme that is involved in maintaining telomeres. These observations need to be confirmed in other populations.Many factors that occur with CFS could contribute to both methylation and telomere length, so the findings are not clear. They do suggest that some of the same biologic factors that are seen in a variety of chronic conditions are also seen in CFS.
