A Never-Ending Immune Battle in ME/CFS? The Regulatory T-cell / Herpesvirus Hypothesis
The failed Rituximab trial might seem like the death knell for autoimmunity in chronic fatigue syndrome (ME/CFS) but it's not - not by a long shot. While the B-cells that Rituximab targeted are at the heart of much autoimmunity, T-cells can also cause autoimmune diseases. They also play a very important role in stopping infections. This interesting paper, conceived and led by a Portuguese researcher named Nuno Sepúlveda, PhD suggests that both options are on the table in ME/CFS. He proposes that a battle between a subset of T-cells called regulatory T cells (Tregs) and herpesviruses may be causing ME/CFS.Nuno Sepúlveda's PhD is in theoretical immunology, and he's on the faculty of the London School of Hygiene and Tropical Medical.The study, the third Sepúlveda has co-authored on ME/CFS, is the tale of both a new hypothesis and a new researcher entering the field.I asked Sepúlveda how he got involved.
This interesting paper, conceived and led by a Portuguese researcher named Nuno Sepúlveda, PhD suggests that both options are on the table in ME/CFS. He proposes that a battle between a subset of T-cells called regulatory T cells (Tregs) and herpesviruses may be causing ME/CFS.Nuno Sepúlveda's PhD is in theoretical immunology, and he's on the faculty of the London School of Hygiene and Tropical Medical.The study, the third Sepúlveda has co-authored on ME/CFS, is the tale of both a new hypothesis and a new researcher entering the field.I asked Sepúlveda how he got involved.
My interest in ME/CFS and the conception of this research came a bit by chance as most things in life. I am a statistician by training but I did a PhD project on theoretical immunology in Gulbenkian Institute for Science in the outskirts of Lisbon. In my PhD theory (supervised by Dr Jorge Carneiro, second author of the paper), I developed mathematical theories on how regulatory T cells regulate autoimmunity throughout life; these cells are thought to be master regulators of the adaptive immune system.In my post-doctoral research, I was a statistical geneticist and a biostatistician doing research in genetics, immunology and epidemiology of tropical and infectious diseases.Along the way I met Luis Nacul and Eliana Lacerda (we are all from the same faculty/institution) who asked me to help them with the statistical analysis of UK biobank data.One day I came across a review paper about autoimmunity and ME/CFS, and I got amazed that no one had done a comprehensive assessment of the role of regulatory T cells on ME/CFS. So I thought to resuscitate my old work on regulatory T cells and give it a go. Then I got hooked up in the field.
We can see how this field widens. Luis Nacul PhD, the senior author of the study, has spent much of his career deeply embedded in ME/CFS. The former leader of the CureME team at the London School of Hygiene and Tropical Medicine, as well as the UK ME/CFS biobank, Nacul is now the Medical and Research Director of the Complex Chronic Diseases Program at BC Women’s Hospital in Vancouver, Canada. He enrolled Sepulveda in taking on ME/CFS.
The Model
"Given this observation, one can hypothesize that these (ME/CFS) patients might be healthy individuals who, by chance, were infected with a microorganism with a strong molecular mimicry to a human protein." Sepúlveda et. al.
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome as a Hyper-Regulated Immune System Driven by an Interplay Between Regulatory T Cells and Chronic Human Herpesvirus Infections. Frontiers in Immunology. eCollection
 It's an inexact science. As with any complex system, the immune system walks a fine line between too much and too little regulation. Too much suppression by the Tregs will impair the immune system's ability to fight off invaders, while too little suppression could result in autoimmunity. Each of us is genetically predisposed one way or the other.People genetically predisposed to more Treg activity would be better at suppressing autoimmunity, but they might also be more prone to letting infections flourish when their Treg cells mistake the pathogenic antigens as self - and call off the immune response.Other individuals predisposed to less Treg activity might be more effective at wiping our pathogens, but more prone to developing autoimmunity. (Since infections, evolutionarily speaking, are more destructive, the authors believe this subset might be more prevalent.)The big question is where do people with ME/CFS fit in? On the one hand, many of their symptoms mimic those found in autoimmune diseases - suggesting they may be immunologically predisposed to have an overly strong immune response to pathogens but are poor regulators of that response.On the other, many people come down with ME/CFS in response to an infection - which suggests they weren't all that good at fighting off pathogens.
It's an inexact science. As with any complex system, the immune system walks a fine line between too much and too little regulation. Too much suppression by the Tregs will impair the immune system's ability to fight off invaders, while too little suppression could result in autoimmunity. Each of us is genetically predisposed one way or the other.People genetically predisposed to more Treg activity would be better at suppressing autoimmunity, but they might also be more prone to letting infections flourish when their Treg cells mistake the pathogenic antigens as self - and call off the immune response.Other individuals predisposed to less Treg activity might be more effective at wiping our pathogens, but more prone to developing autoimmunity. (Since infections, evolutionarily speaking, are more destructive, the authors believe this subset might be more prevalent.)The big question is where do people with ME/CFS fit in? On the one hand, many of their symptoms mimic those found in autoimmune diseases - suggesting they may be immunologically predisposed to have an overly strong immune response to pathogens but are poor regulators of that response.On the other, many people come down with ME/CFS in response to an infection - which suggests they weren't all that good at fighting off pathogens.
The Third Way - the ME/CFS Way?
There is a third way, though - a kind of a worst of both worlds way - and that's what this group's mathematical modeling, the first of its kind done in ME/CFS, uncovered. If the authors are right it could explain why people with ME/CFS are in such a fix.
The Herpesviruses
The authors demonstrated how such a situation could happen by modeling the effect of herpesviruses on the T regulatory cells in ME/CFS in different immunological contexts.HHV-6Using HHV-6, the authors proposed that an ME/CFS state could occur when a smoldering infection is responded to by a T-cell clone with a high potential for producing an autoimmune state.T-cell clones are populations of T-cells which contain both T regulatory cells and T effector (helper) cells. Because their composition reflects the immune milieu around them, a T-cell clone with a high autoimmune potential reflects a T-cell clone existing in an environment loaded with self-antigens; i.e. antigens from a pathogen which look like they come from humans. In this example, an HHV-6 infection producing many self-like antigens is present.Because the infection is smoldering and the viral load is low, though, the full T-helper immune response which would serve to stop the infection is not initiated. Nor do the T regulatory cells fully step in to ward off an autoimmune response.Instead, the virus, replicating slowly, triggers both responses. As the Treg cells tamp down the chronic immune response, they also shut down the the cytotoxic NK cells. With the immune system not geared up in either direction, the smoldering infection continues in perpetuity causing high energy costs as well as inflammation and fatigue, and there you have it - a metabolically exhausting state of inflammation and fatigue; i.e. ME/CFS.
Epstein-Barr Virus
A similar situation may occur with EBV when a Treg clone with a high autoimmune potential co-occurs with a low T-cell killing rate. Instead of a blatant autoimmune response that racks the body, or an effective response to the pathogen, you get partial amounts of both: you get both a sucky immune response to a pathogen AND an autoreactive reaction. If the authors are right it's no wonder ME/CFS is such a puzzle and so difficult to treat.How do the authors believe this shows up biologically? In a high density of and increased percentage of Treg cells in ME/CFS patients compared to healthy controls and people with autoimmune diseases. That's actually what they see in their ME/CFS patients in their lab.
Different Roads Taken
Interestingly, the authors believe both autoimmune diseases and ME/CFS start off the same path - both are triggered by the cumulative effects of an autoreactive response to a common viral infection - but then both flit off on different paths.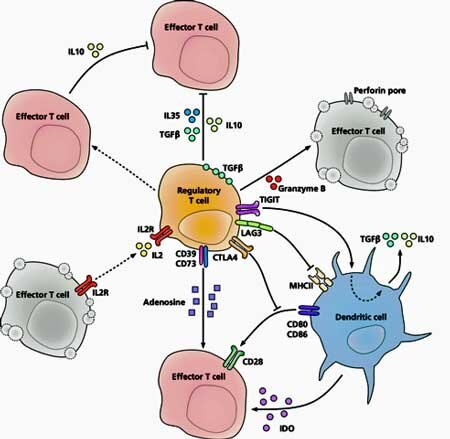 Herpesviruses may be setting off autoimmune reactions in both autoimmune diseases and ME/CFS, but in ME/CFS the Treg cells kick in to dampen down the autoimmune response.Unfortunately, as they're doing that they're also bollixing up the immune response to the pathogen - leaving ME/CFS patients in the strange state of both defending against a pathogen and trying to dampen down an autoimmune response at the same time - a metabolically exhausting situation.The authors believe that a genetic predisposition affecting T-cells would probably be present in ME/CFS, and pointed to genetic polymorphisms that have been found. Defective T-cell responses to Epstein-Barr Virus have also been found in ME/CFS. Further study of the T-cell repertoires in ME/CFS are needed, though, as well as studies to validate whether Treg density and percentages are increased in ME/CFS.T-cells - perhaps the single most impactful immune cell in the body - have become the focus of interest of a number of other ME/CFS researchers including Derya Unutmaz at the Jackson Labs, and Mark Davis at Stanford.Smoldering viral infections have also become a hot topic in ME/CFS. Bob Naviaux and Bhupesh Prusty propose a smoldering HHV-6 infection, and Marshall Williams proposes a smoldering Epstein-Barr infection may be present in ME/CFS, as well.
Herpesviruses may be setting off autoimmune reactions in both autoimmune diseases and ME/CFS, but in ME/CFS the Treg cells kick in to dampen down the autoimmune response.Unfortunately, as they're doing that they're also bollixing up the immune response to the pathogen - leaving ME/CFS patients in the strange state of both defending against a pathogen and trying to dampen down an autoimmune response at the same time - a metabolically exhausting situation.The authors believe that a genetic predisposition affecting T-cells would probably be present in ME/CFS, and pointed to genetic polymorphisms that have been found. Defective T-cell responses to Epstein-Barr Virus have also been found in ME/CFS. Further study of the T-cell repertoires in ME/CFS are needed, though, as well as studies to validate whether Treg density and percentages are increased in ME/CFS.T-cells - perhaps the single most impactful immune cell in the body - have become the focus of interest of a number of other ME/CFS researchers including Derya Unutmaz at the Jackson Labs, and Mark Davis at Stanford.Smoldering viral infections have also become a hot topic in ME/CFS. Bob Naviaux and Bhupesh Prusty propose a smoldering HHV-6 infection, and Marshall Williams proposes a smoldering Epstein-Barr infection may be present in ME/CFS, as well.
A Further Widening Field
The "widening" of the ME/CFS field is continuing with Sepúlveda. When I asked him what he's working on next he reported he was bringing new researchers (and new funders) into the field as well. His research into this possible aspect of ME/CFS is continuing full-bore.
Currently I have a PhD student working full time on a project extending some ideas about the role of regulatory T cells on ME/CFS. This project is funded by the Portuguese Foundation for Science and Technology and my student is doing his research in the Molecular Medicine Institute in Lisbon. I am also trying to find/identify candidate molecular mimicries between viruses and human proteins that could explain ME/CFS.

